When we talk about our instrumentation, it’s with the goal of measuring various isotopes… but not all isotopes are created (or destroyed) equally! This is a summary of isotopes in terms of longevity, abundance, and elemental behavior. When people think of isotopes they often think of nuclear plants… but what makes certain isotopes useful for nuclear energy and others useful for studies of our planet? Great question! We’re so happy you asked…
To start off let’s make sure we’re on the same page… remember high school chemistry? Maybe you do or maybe you don’t (or maybe you forget it on purpose)! An element on the periodic table contains protons, neutrons, and electrons, and is defined by the number of protons it has. This value is called its “atomic number” and is often abbreviated as “Z”. An isotope of an element has the same number of protons, but can vary in its number of neutrons. For example, the element oxygen has 8 protons, but can have 8, 9, or 10 neutrons; therefore, oxygen has 3 isotopes (technically there are others but they are present in very tiny quantities). When we describe oxygen isotopes, we refer to them as oxygen 16, 17, and 18 (8 protons plus however many neutrons). Oxygen 16 and 18 are used most frequently in practical applications because there’s only a small amount of 17O… but we’ll get to that later…

Longevity: Now that we know how an isotope is defined, how do we use them? It depends on what information we want. Isotopes can be broken into two primary categories: stable and radiogenic. Stable isotopes are just as they sound – very stable! They do not undergo any type of decay to other elements. Radiogenic isotopes DO undergo decay, and this is where you may have heard of terms like half-lives and radioactivity. And if you’re wondering… yes, we have mass spectrometers that can measure both!
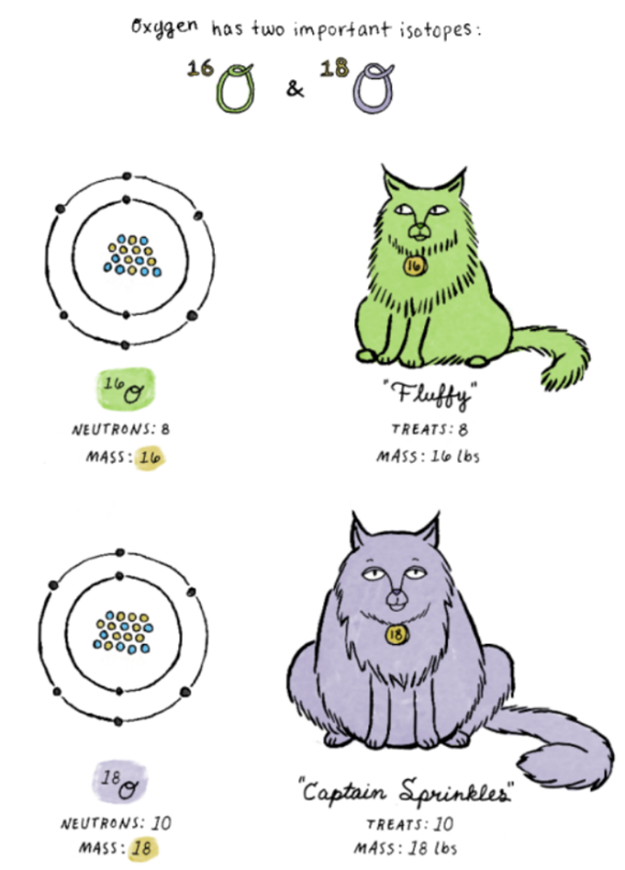
Now back to the original question – what the heck do we use these for? Well, stable isotopes can be used in a variety of ways. Most commonly, stable isotopes are used as a “tracer” to figure out the history of a rock or the paleoclimate in which the rock originally formed (disclaimer: we aren’t using stable isotopes in this study). No dating is involved here because nothing is decaying at any known rate. This “tracer” works because these stable isotopes are mass dependent. Think back to our oxygen example… an isotope of 18O is a smidge heavier than the isotope of 16O. Why? Because those two extra neutrons add weight (mass)… See the cartoon of the cats (below) to help visualize this concept. And if you had an extra slice or two of pizza last night, you personally might be able to relate to this concept!
In addition to stable isotopes, there’s also radiogenic isotopes which decay over time and can be used to date… and by date we mean learn about time, not a romantic courtship! You may have heard of half-lives and techniques like carbon-dating without any context. Radioactivity is the result of an inherent instability in the number of protons and neutrons in an element which causes it to turn into other elements and/or isotopes over time. A half-life is how long it takes for half of an isotope of an element to decay away… into something else.
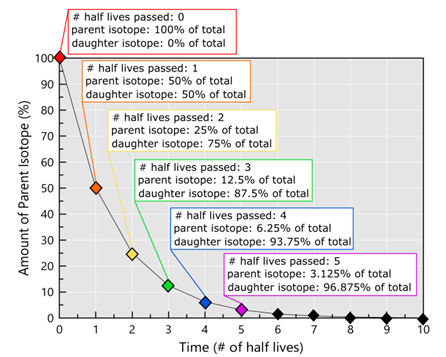
Imagine you have an isotope that has a half-life of 100 years. In 100 years, only 50% of that isotope is left, and after 200 years only 25% of that isotope is left. It has experienced two half-lives, and a half of a half is a quarter. After ~7 half-lives there’s <1% of the parent isotope left and most instrumentation cannot detect such small amounts of material… You can get a sense of this from the plot below.
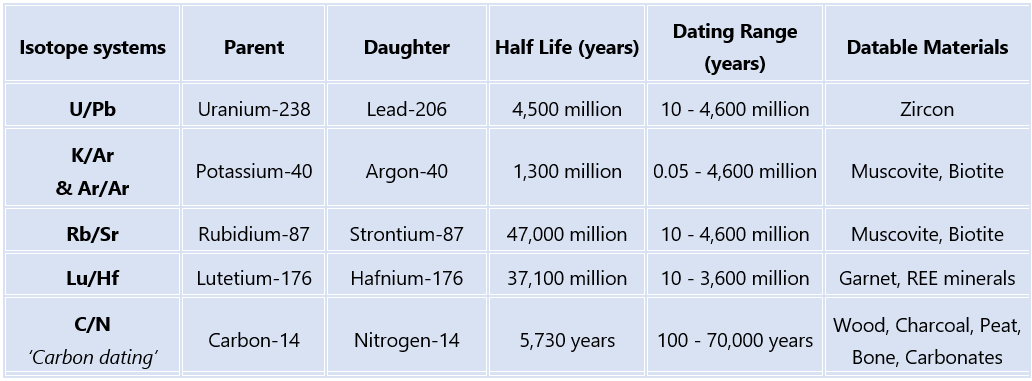
One of the most well-known applications of this technique is carbon dating (14C), but there is also K/Ar, Ar/Ar, U/Pb, Rb/Sr, Lu/Hf just to name a few. However, in order to use one of these methods to date something, you need to meet two big requirements:
1. The parent isotope needs to be present in the material being dated (if the element isn’t there, there’s nothing to decay!)
2. The half-life of the system needs to be an appropriate length of time for the material being dated (we need to be able to accurately measure both parent and daughter isotopes… so enough parent isotope remaining, and daughter isotope produced)
Using our example of 14C (commonly known as ‘carbon dating), let’s test your knowledge! Could 14C be used to date a volcanic rock that is estimated to be between 20-40 million years old?
*cue gameshow music*
What do you think? And is that your final answer? Okay… we’ll just tell you! You cannot use the 14C method to date a 20-40 million year old volcanic rock because it would fail both requirements! This is because 1. carbon is not present in most volcanic rocks, and 2. the half-life of 14C is only 5,715 years and thus too short for the rocks you’re interested in. Using the 14C system, anything older than ~40,000 years doesn’t have enough of the parent isotope left to measure! Think about trying to measure the distance between Seattle to Boston with a ruler; your unit of measurement is wrong for the scale you’re interested in.
This brings us to another way to think about isotopes, their abundance on earth! Remember that early reference to oxygen isotopes, specifically oxygen 17 (17O)? Worth mentioning that these are the 3 isotopes of oxygen that make up almost all oxygen. However other isotopes of oxygen do exist… they are radioactive and include 11O to 26O. In addition to volumetric abundance, it’s worth thinking about how this can be the result of very short-lived half-lives – the shortest-lived isotope is 12O with a half-life of 5.8 x 10-22 seconds!
Finally, let’s consider how the type of element or elemental behavior impacts our approach to measuring isotopes! If you think about the periodic table, we cannot measure helium or argon, both noble gases – the same way we measure potassium and rubidium, both alkali metals. Since different types of elements can behave differently, some require different measurement methods. This is the reason the K/Ar method evolved into the Ar/Ar method! It’s not easy to measure K isotopes at the same time as Ar isotopes… and doing so results in inaccurate measurements. Now using Ar/Ar, we add the step of a nuclear reactor to irradiate our sample and bombard it with neutrons which turn the parent isotope of 40K into 39Ar. This allows us to measure the parent and daughter isotopes simultaneously using an instrument like the NGX, since they are both noble gases!
As you can see, there are lots of different ways to classify isotopes. And while only some isotopes are radiogenic… we think all isotopes are rad!
If you’ve got any thoughts, questions or comments about the basics of isotope ratios and their measurement – please let me know, I’d be keen to hear (emily.cahoon@isotopx.com).
